Providing patients with safe and effective healthcare solutions is the principal goal of every pharmaceutical manufacturing organization. The success of these organizations depends on their ability to maintain SQUIPP: safety, quality, identity, purity, and potency of the products. SQUIPP is closely linked to the effectiveness of way of working and control strategies implemented to ensure the manufacturing operations are in control, so the pharmaceutical products that go into the market are safe and effective.
Variations in manufacturing operations, raw materials, and equipment reliability often disturb process performance, which can potentially impact SQUIPP, along with product yield (which falls outside of SQUIPP) and quality attributes. In the pharmaceutical and biologics manufacturing industries, the current state of data analytics for process performance monitoring largely involves descriptive analytics, which focuses on what happened, and diagnostic analytics, which helps to understand why something happened.
Descriptive and diagnostic analytics are used to solve manufacturing issues, with the penalty of longer reaction time. The application of these two reactive modes of data analytics is highly matured and widely used for problem solving at Sanofi’s Toronto, Canada, site to support process investigations. However, these methods are used reactively, initiated upon requests from process experts when a process- or product-related issue is identified. As a result, potential negative shifts in process performance and quality attributes may get flagged with delay, sometimes requiring time-consuming investigations.
Thus, the data science team at Sanofi’s Toronto, Canada, site began an initiative to move toward prescriptive analytics. It sought to increase timely process monitoring and diagnosis and to move from a reactive to a proactive mode of operation that would enhance data analytics capabilities and increase efficiencies. This initiative aims to: (1) identify process performance shifts proactively by implementing live analytical models to actively detect issues and alert experts and (2) augment data analytics activities to prescriptive models (i.e., to enable data-driven decisions on what actions are needed to avoid an impending negative effect).
The current reactive modes of data analytics (descriptive and diagnostic analytics) have successfully helped solve manufacturing performance and product-quality issues. However, identifying the root cause and applying solutions usually happens several impacted batches later. The new prescriptive analytics solution has the potential to reduce that reaction time considerably, which would allow faster recovery of manufacturing operations and avoid potential impacts to the business.
Figure 1 shows an illustrative current state and proposed future state. The future state uses knowledge management to perform automated and autonomous analytics and leverage previous learnings to arrive at a rapid solution. Subject matter experts (SMEs) can then take action to remediate the problem.
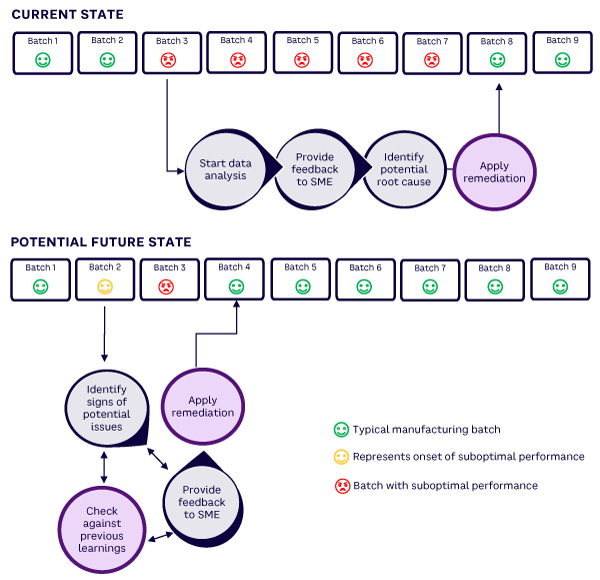
support for manufacturing (for illustration purposes only)
[For more from the authors on this topic, see: “Sanofi’s Move Toward Prescriptive Data Analytics.”]
Disclaimer: This work was funded by Sanofi. There are no potential conflicts of interest that the authors are aware of. Ramila Peiris and Hossein Sahraei equally contributed to drafting the content, while Olivier Moureau and Natalija Jovanovic provided feedback to improve the content. The authors are Sanofi employees and may hold shares in the company.